Beijing CorreGene Biotechnology Co., Ltd. (hereinafter referred to as "CorreGene"), a strategic partner of Shenzhen Eureka Biotechnology Co., Ltd. (hereinafter referred to as "EurekaBio"), obtained the Investigational New Drug (IND) implied license for its self-developed T cell receptor-engineered T cell (TCR-T) product, "CRTE7A2-01 TCR-T Cell Injection", from the Center for Drug Evaluation (CDE), NMPA on November 21, 2023. EurekaBio extends the warmest congratulations to CorreGen on this important milestone!
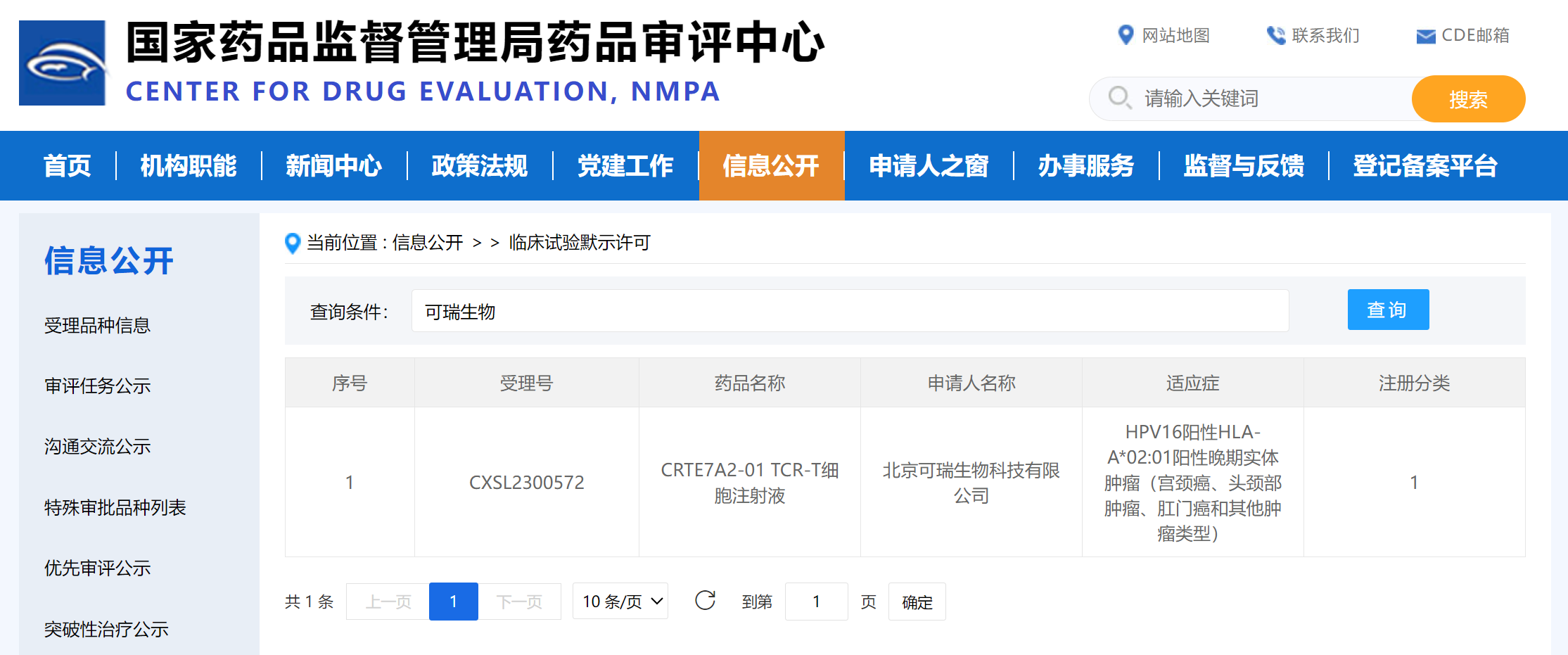
CorreGene has taken advantages of technological innovation to establish a systematic TCR research and development (R&D) platform, solve a series of technical difficulties in TCR cloning and optimization, and efficiently carry out high-throughput development of innovative TCR drugs. CorreGene has also substantially improved the success rate and efficiency of optimizing TCR affinity based on the independently created SMART-TCR affinity optimization platform, and built a perfect R&D technology platform for soluble TCR-based protein drugs. Moreover, the highly innovative R&D technology platform is combined with the mature industrialization platform for process development, analysis and quality control to develop numerous multi-target product lines for various solid tumors and viral infections. CRTE7A2-01 TCR-T Cell Injection, independently developed by CorreGene, targets human papillomavirus 16 (HPV16) E7 envelope protein, with good safety and excellent anti-tumor activity, which is intended to be developed for the treatment of HPV16-positive advanced cervical cancer, head and neck tumors, and anal cancer.
▲Photo source: official website of CorreGene
As an upstream core supplier of cell and gene therapy (CGT), EurekaBio is extremely honored to provide automatic solutions to cell preparation for "CRTE7A2-01 TCR-T Cell Injection" of CorreGene. The CellSep®PRO automated closed cell processing system developed by EurekaBio accelerates the launching of CorreGene's TCR-T products with its innovative production process and stable processing capacity, thus benefiting more patients.
CorreGene is an important partner of EurekaBio, while the automated cell processing equipment of EurekaBio can fully cooperate with CorreGene in registering clinical studies and developing processes of new lines. Both parties will continue promoting the development of advanced therapies in the future. Relying on the powerful R&D and transformation ability of CorreGene in innovative immune cell therapies, as well as the CellSep® automated closed cell processing systems and EuLV® lentiviral vector production system based on stabile cell lines of EurekaBio, both companies will give full play to their advantages to enhance the accessibility of their products and enable the cell therapy drugs to benefit all global patients.
Adhering to the vision of "empowering CGT and solving the most complicated problems in the industry", EurekaBio has achieved cross-disciplinary integration and technological innovation in automation technology, artificial intelligence and life sciences, overcoming the difficulties in core technology and core equipment during the R&D and production of CGT drugs, providing CGT customers with innovative overall solutions to cell production and solutions to viral vector production, and assisting in the industrialization and commercialization of CGT drugs.
Related reading
IND approval for the first immune cell therapy product of CorreGene
(link: https://mp.weixin.qq.com/s/habL5SnxzDRtHRsBsFUN2g)
About CorreGene
Founded by a number of Peking University alumni and returned overseas students, CorreGene is dedicated to developing innovative T-cell receptor (TCR)-based drugs. It has established a systematic TCR R&D platform through technological innovation to solve a series of technical difficulties in TCR cloning and optimization, which is able to efficiently carry out high-throughput development of innovative TCR drugs. CorreGene has also substantially improved the success rate and efficiency of optimizing TCR affinity based on the independently created SMART-TCR affinity optimization platform, and built a perfect R&D technology platform for soluble TCR-based protein drugs. Moreover, the highly innovative R&D technology platform is combined with the mature industrialization platform for process development, analysis and quality control to develop numerous multi-target product lines for various solid tumors and viral infections. In the future, CorreGene will develop a variety of drugs with indications for tumors and chronic infections by virtue of TCR-T cell drugs and TCR-based protein drugs, aiming at the tera-scale disease market and committing to becoming an outstanding enterprise of TCR-based immunotherapy.
About EurekaBio
EurekaBio is an upstream core supplier of CGT, focusing on the R&D of key technologies and processes with the goal of decreasing costs and increasing benefits. As a leading enterprise of automated cell preparation equipment in China, EurekaBio has independently researched, developed and launched a series of CellSep® cell processing systems, together with sterile connecting devices, heat sealing machines and wave bioreactors, to provide a comprehensive solution for the preparation and manufacturing of cell therapy products. Meanwhile, EurekaBio has made innovations in developing the EuLV® system for large-scale manufacturing of lentiviral vectors based on stable cell lines, which has broken the technical bottleneck in large-scale manufacturing of lentiviral vectors, substantially enhanced the production efficiency of lentiviral vectors and the homogeneity of products, and significantly reduced the manufacturing costs.







.jpg)




