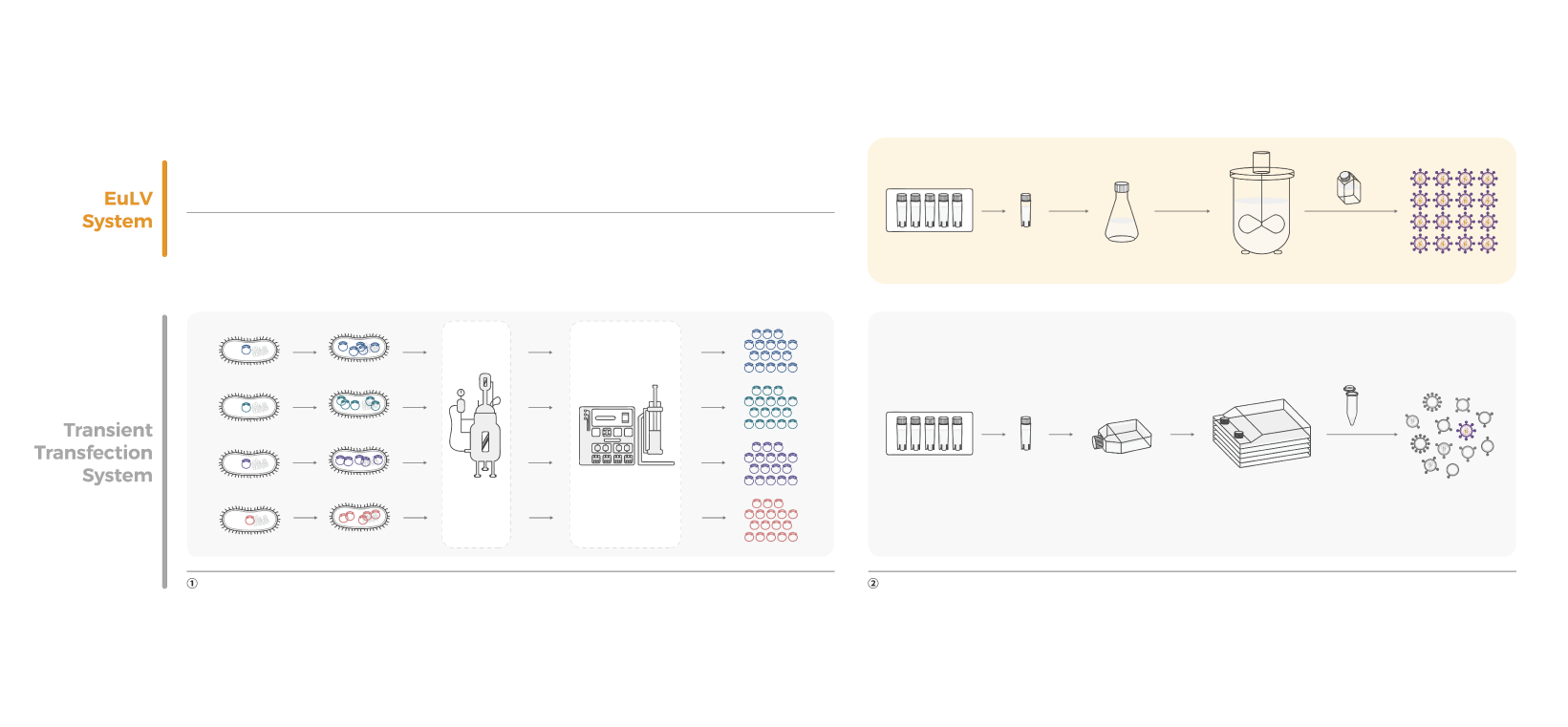
The EuLV™ system, a stable lentiviral vector production system, employs inducible suspension-based producer cell lines in the high cell-density, serum-free media to produce lentiviral vectors.
The EuLV™ stable system emerges as the ideal option for large-scale LVV manufacturing because of:
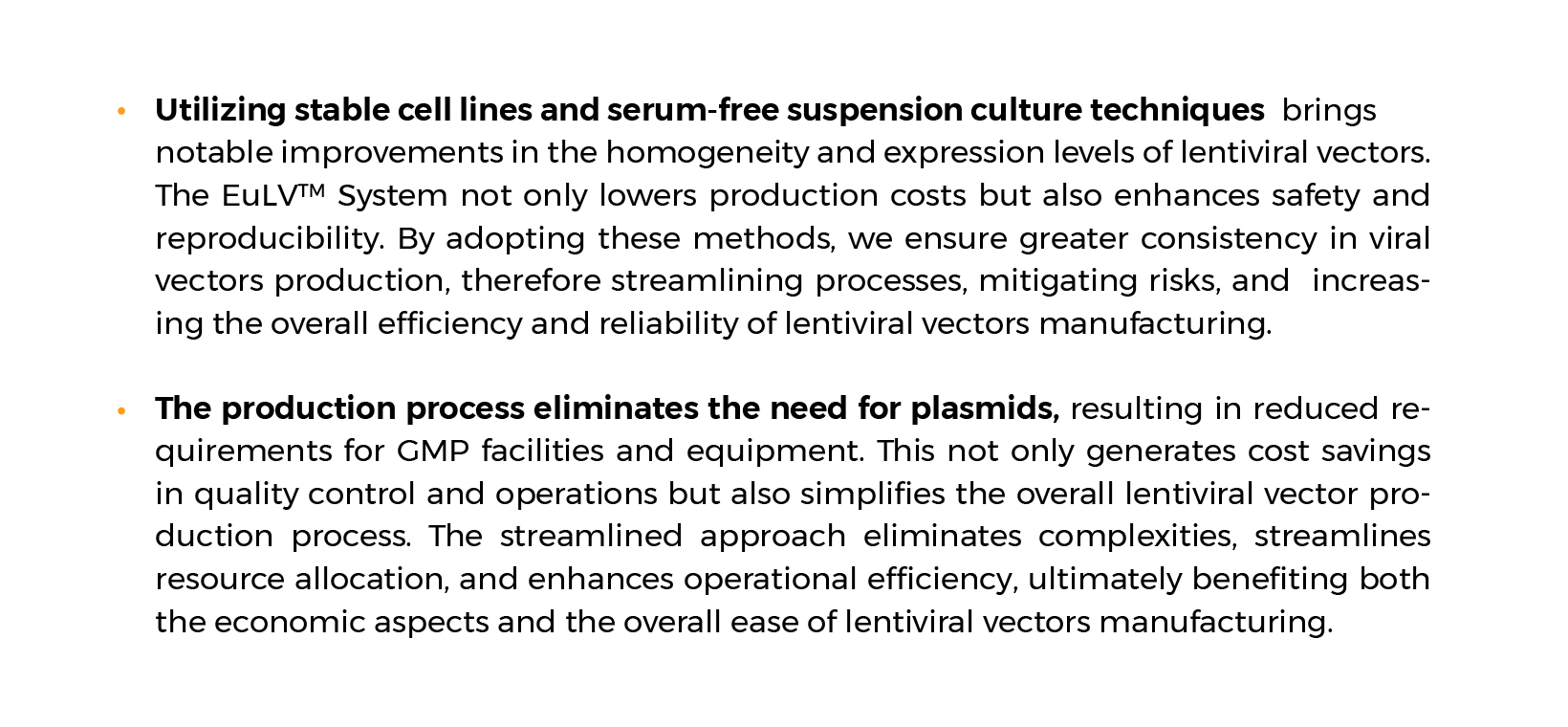
- Streamlined vector production: No addition of raw materials such as DNA plasmids and transfection reagents.
- Scalable vector production: the serum-free suspension cell culture process is easier to scale up.
- Higher titer yield and simpler process development.
- Reproducible vector production: To produce safer viral vectors with low batch-to-batch variability.

Advantages
-50 %
A 50% Reduction in Production Procedure
-80 %
An 80% Reduction in Production Costs
100 Fold
A 100-Fold Increase in Production Efficiency
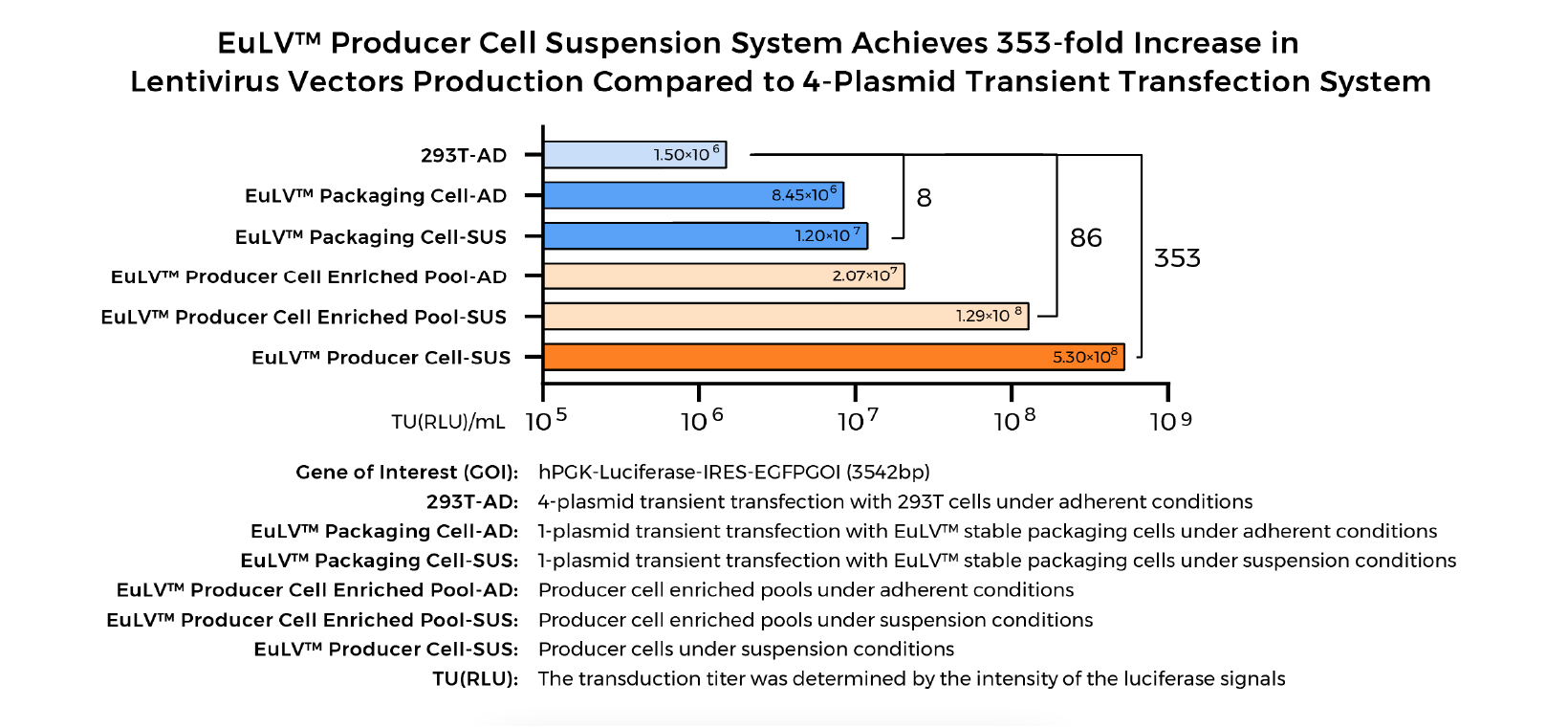
EuLV™ Stable LVV System Superior to Transient Transfection System
EuBioX - Fully Automated Cell Screening System
The EuBioX, an in-house developed fully automated cell screening system, plays an instrumental role in the EuLV™ stable producer cell line development. The EuBioX system not only streamlines the process of the single clone selection, allowing to quickly and accurately verify the desired single clones but also significantly reduces the screening workload while enhancing the accuracy of the lentiviral producer cell line screening process.
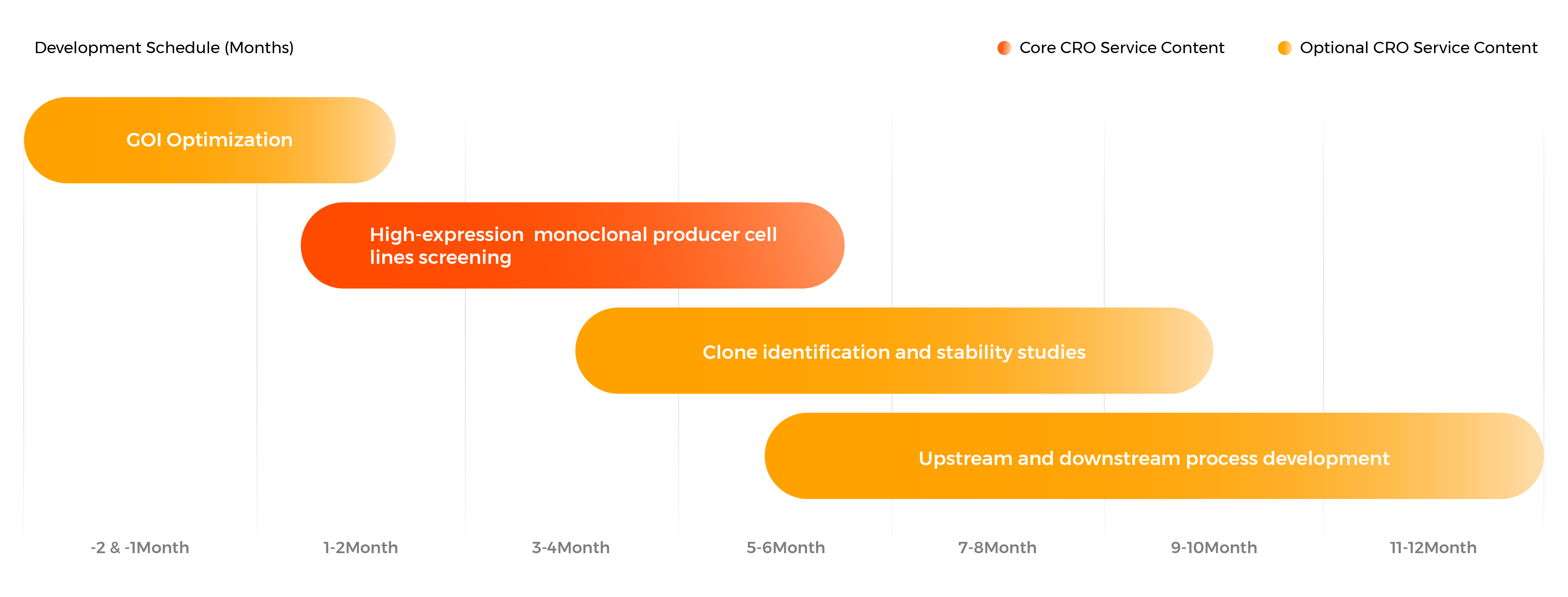
EuLV™ Producer Cell Line Development Process







.jpg)