
May 16, 2025 – Cytiva and Eureka Biotechnology, Ltd (EurekaBio) today announced a strategic partnership to advance lentiviral vector (LVV) manufacturing in China. The collaboration centers on EurekaBio’s proprietary EuLV™ lentiviral vector stable producer cell line production system with the goal of combining the companies’ strengths and resources to drive innovation in viral vector manufacturing for the rapidly growing cell and gene therapy (CGT) sector.
As CGT technologies progress, the demand for reliable, cost-effective viral vector manufacturing solutions has become increasingly critical. EurekaBio’s EuLV™system provides a robust alternative to conventional transient transfection methods, offering a scalable, efficient, and economically viable approach to LVV production. Key advantages of the EuLV™system include:
• Simplified Process: Eliminates the need for GMP-grade plasmids through a stable producer cell line approach.
• Cost Efficiency: Reduces reliance on plasmids and transfection reagents, while delivering high productivity.
• Scalability: Enables high-density suspension culture, ideal for large-scale manufacturing.
• Consistent Quality: Uses well-characterized producer cells with high uniformity and minimal batch-to-batch variation.
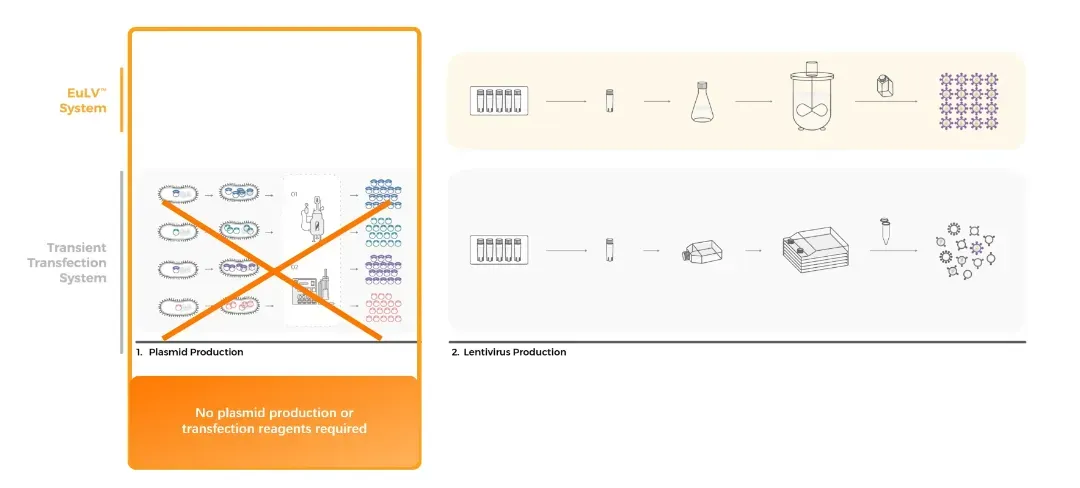
Through this strategic alliance, Cytiva and EurekaBio will empower biopharmaceutical enterprises to integrate EurekaBio’s proprietary EuLV™ system into their workflows, enabling scalable, GMP-compliant manufacturing of advanced cell and gene therapies. Under the agreement, Cytiva will secure exclusive sub-licensing rights to the patented EuLV™ technology and execute comprehensive licensing services across China. The parties will collaborate to execute milestone-driven licensing agreements for biopharmaceutical enterprises, ensuring clients secure robust and legally enforceable intellectual property protections aligned with global regulatory standards.
Localized Cell Banking for Accelerated Drug Development
Cytiva’s China Innovation Center will leverage its localized process development expertise and end-to-end CGT industry experience to establish and maintain Master Cell Banks (MCB) and Working Cell Banks (WCB) for the EuLV™packaging cell line in China. These cell banks comply with both Chinese and U.S. GMP standards, supporting preclinical studies, clinical trials, regulatory submissions, and commercial production. By offering shared, ready-to-use cell banks, the collaboration aims to reduce costs and accelerate drug development for biopharma companies.
Li Lei, President of Cytiva China, stated, “Cytiva is committed to advancing China’s biopharmaceutical industry through partnerships with local innovators. We will deepen collaboration with domestic pioneers to empower industry growth and establish China as a global innovation hub.”
Yuan Ming, General Manager of Cytiva China’s GenomicMedicine Business Unit, added, “This partnership enables us to provide CGT companies with economical, efficient, and stable lentiviral production solutions. Cytiva will continue to engage deeply in China’s CGT ecosystem, driving high-quality industrial development and accelerating the translation of innovation into commercialization.”
Ma Marl, CEO of EurekaBio, emphasized, “CGT breakthroughs demand both technological rigor and open collaboration. Partnering with Cytiva marks a critical step in aligning our proprietary innovations with international standards. Starting with EuLV™ technology, we aim to optimize production processes, lower treatment costs, and bridge the gap between R&D and industrialization. Together, we will deliver stable, reliable solutions to benefit patients globally.”
EurekaBio, guided by its mission to “Expand the Frontiers of Treatment,” focuses on advancing CGT technologies and industrialization. By combining its EuLV™ system with Cytiva’s global resources, the collaboration aims to overcome technical and cost barriers in viral vector manufacturing, accelerating the translation of innovative therapies from lab to clinic.
In the era of CGT industrialization, true progress requires both technological excellence and ecosystem vision. This partnership exemplifies EurekaBio’s commitment to innovation and collaboration—validating its technology against international benchmarks while addressing local industry needs. By relentlessly optimizing processes and fostering open partnerships, EurekaBio reaffirms its pledge to “reduce costs, enhance efficiency, and deliver life-changing therapies to patients worldwide.”
About EurekBio
EurekaBio is a leading upstream technology provider in the CGT space, focused on the independent development of core technologies and specialized equipment. By integrating expertise across computer science, electronics, nanomaterials, and chemical engineering with life sciences, Eureka Bio is advancing next-generation tools and technologies to address critical challenges in gene therapy, cell therapy, and pharmaceutical R&D—pushing the frontiers of therapeutic innovation.
About Cytiva
Cytiva is a global life sciences leader, employing approximately 15,000 people across more than 40 countries. Dedicated to advancing breakthrough technologies and accelerating life-changing therapies, Cytiva partners with researchers, biotech developers, and manufacturers to support development in biopharmaceuticals, cell and gene therapies, and mRNA-based technologies. By enhancing the capability, speed, and flexibility of bioprocessing, Cytiva helps bring transformative medicines to patients around the world.








.jpg)




